The NLRP3 inflammasome is the most thoroughly studied of the inflammasomes, and represents a valuable target in the clinical evaluation and treatment of systemic autoinflammatory diseases (SAIDs). We’ve summarised the rapidly progressing field of NLRP3 inflammasome regulation and its wider implications, both in the laboratory and in the clinic.
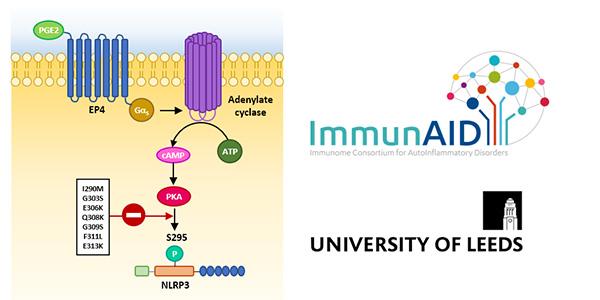
The protein kinase A signaling pathway is just one of the many physiological pathways which regulate NLRP3 activity; it has been reviewed, among many others, by the team.
Understanding the mechanisms underlying SAIDs is a vital aspect of improving their classification and treatment. Inflammasomes, which are large cytosolic multiprotein complexes that mediate proinflammatory cytokine production, pyroptosis and alarmin release, have been implicated in the pathogenesis of numerous SAIDs. Inhibition of inflammasomes, or of the cytokines they release, have shown promise as treatments for these conditions. The NLRP3 inflammasome is the best characterised inflammasome. It has been identified as a common mechanism underlying a broad spectrum of disease ranging from monogenic autoinflammatory conditions, such as cryopyrin-associated periodic syndromes (CAPS), through to complex innate immune-mediated disease such as inflammatory bowel disease and Behçet syndrome.
In a recent article published in Genes and Immunity, the team from Leeds describes the complex molecular machinery involved in the activation and regulation of the NLRP3 inflammasome. Developing an in-depth understanding of these processes is integral to the study of SAIDs and has numerous implications, including the clinical evaluation of undifferentiated SAIDs and the development of molecular agents to modulate NLRP3 inflammasome function, both in the laboratory and the clinic.
“This review spans a range of our research interests, including the study of physiological inhibitors in humans, disease-related biomarkers and the structural biology of inflammasomes”, says author Emily Caseley at Leeds. “It also represents the first collaboration with François Rodrigues*, who is particularly interested in clinical aspects of inflammasome inhibition”.
Finally, the article provides a thorough overview of NLRP3 inhibitors in clinical trials and their therapeutic potential.
“The study of NLRP3 inflammasome regulation and inhibition has the potential to revolutionise the field of SAIDs research. We hope that this publication has highlighted the importance of inflammasome regulation in SAIDs, as well as emphasising some promising therapeutic avenues for future work and collaboration within ImmunAID”, says Michael McDermott, last author of the publication. We hope too, and are even pretty confident about it!
*: François Rodrigues works for the « Centre de Référence des Maladies Auto-inflammatoires et des Amyloses d’origine inflammatoire », Sorbonne University, Paris.



